-
PDF
- Split View
-
Views
-
Cite
Cite
Yoichi Nakamura, Hideo Kunitoh, Kaoru Kubota, Ikuo Sekine, Tetsu Shinkai, Tomohide Tamura, Tetsuroh Kodama, Minako Sumi, Shigeru Kohno, Nagahiro Saijo, Platinum-based Chemotherapy with or without Thoracic Radiation Therapy in Patients with Unresectable Thymic Carcinoma, Japanese Journal of Clinical Oncology, Volume 30, Issue 9, September 2000, Pages 385–388, https://doi.org/10.1093/jjco/hyd108
Close - Share Icon Share
Abstract
Background: Thymic carcinoma is a rare mediastinal neoplasm with poor prognosis. Although the clinical benefit of chemotherapy for thymic carcinoma is controversial, cisplatin-based chemotherapy with or without radiation therapy is ordinarily adopted in advanced cases. We evaluated the clinical outcome of platinum-based chemotherapy with or without radiation therapy in unresectable thymic carcinoma patients.
Methods: Ten patients with unresectable thymic carcinoma were treated with platinum-based chemotherapy with or without radiation therapy in the National Cancer Center Hospital between 1989 and 1998. We reviewed the histological type, treatment, response and survival of these patients.
Results: Four of the 10 patients responded to chemotherapy and both the median progression-free survival period and the median response duration were 6.0 months. The median survival time was 11.0 months. There was no relationship between histological classification and prognosis.
Conclusions: Platinum-based chemotherapy with or without thoracic radiation is, regardless of tumor histology, marginally effective in advanced thymic carcinoma patients, giving only a modest tumor response rate and short response duration and survival.
Received March 29, 2000; accepted July 11, 2000.
INTRODUCTION
Thymic carcinoma is a rare epithelial tumor of the thymus that differs from thymoma in its malignant features (1,2). In general, thymic carcinoma is characterized by extensive local invasion and distant metastasis, an aggressive course and a poor prognosis.
Although resection is the first line of treatment of thymic carcinoma in most cases, radiation and/or cisplatin-based chemotherapy are also utilized in cases that are unresectable (2–7). The clinical benefit of chemotherapy for unresectable thymic carcinoma remains controversial. Some investigators have documented no significant benefit of chemotherapy for thymic carcinoma (2,3), but others have reported occasional complete response to chemotherapy and have advocated a cisplatin-based combination (4,5). We report the response and clinical course of 10 cases of unresectable thymic carcinoma that were treated with platinum-based chemotherapy with or without radiotherapy in the National Cancer Center Hospital between 1989 and 1998.
MATERIALS, METHODS AND STATISTICS
We treated 10 cases of unresectable primary thymic carcinoma. In each case, histological diagnosis was made using needle biopsy specimens. The diagnostic criteria for primary thymic carcinoma were based on the existence of an anterior mediastinal tumor at the thymic region showing the histocytological features of thymic malignancy, as proposed by Tsuchiya et al. (8). Formalin-fixed and paraffin-embedded tissues were used for the histopathological examination and were reviewed by two pathologists at the Department of Pathology at the National Cancer Center. Pretreatment evaluation of the tumor was done by physical examination, chest X‐ray film, chest and brain computed tomography, abdominal ultrasonography and bone scan.
Evaluation of response was conducted by physical examination, chest X-ray and chest computed tomography after each treatment. A complete response (CR) was defined as the complete disappearance of all objective evidence of disease lasting at least 4 weeks. A partial response (PR) was defined as a decrease of at least 50% in the sum of the perpendicular diameters of measurable lesions at 4 weeks or later. Progressive disease (PD) was defined as an increase of at least 25% in tumor size or the appearance of new lesions. All other circumstances were classified as no change (NC).
Survival distributions were computer-generated using the Kaplan–Meier product-limit method and curve comparison was carried out by the log-rank test.
RESULTS
Patient Profiles
Ten patients were treated in our hospital (Table 1), consisting of six men and four women whose ages ranged from 27 to 71 years with a median of 50.5 years. The histological subtypes of thymic carcinoma were four squamous cell carcinoma and six poorly differentiated carcinoma. Five patients had locally advanced disease and the other five had metastatic disease at the time of presentation. None of them exhibited any paraneoplastic syndromes such as myasthenia gravis or hematological abnormalities.
Treatment
All patients received platinum-based chemotherapy (Table 2). The primary tumor of patient 1 was operable after response to chemotherapy and the tumor was resected completely. Patients 4 and 7 received only one course of chemotherapy, because patient 4 refused further therapy and patient 7 required emergency thoracic radiotherapy because of tumor invasion to the heart and great vessels. Seven patients were treated with irradiation of 6–56 Gy to primary tumors after chemotherapy (Table 2). The total doses were given in multiple fractions of 2 Gy per day. Patient 4 developed superior vena cava syndrome because of the rapid progression of the tumor and died of it despite the start of thoracic radiation.
Response, Survival and Progression-free Survival
Four of the patients responded to each chemotherapy (Table 2). Three were poorly differentiated carcinoma and one was squamous cell carcinoma. There was no significant difference in response between these two groups (p = 0.57, Fisher’s exact test). At the time of this analysis, nine deaths have been recorded and all the causes of death were progressive tumor. The median survival time (MST) was 11.0 months and the 1‐year survival rate was 45.7%. The median progression-free survival period was 6.0 months and the median response duration was also 6.0 months. With regard to the sites of tumor recurrence, there were seven mediastinal recurrences, six lung metastases, four bone metastases, two liver metastases and one lymph node metastasis. Eight patients had multiple foci of tumor recurrence (Table 3).
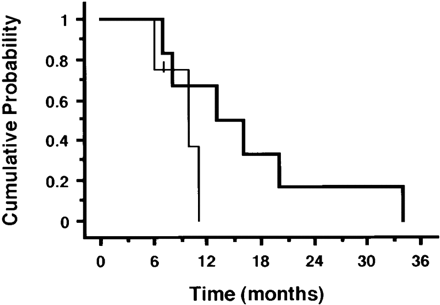
Fig. 1 shows the cumulative survival curve for the patients according to the histological classification. The MST for patients in the low-grade and high-grade groups were 10 and 13 months, respectively.
DISCUSSION
Thymic carcinoma is a thymic epithelial neoplasm which has been proposed by several investigators to have malignant features and which includes various histological subtypes that closely resemble carcinomas seen in other organs (1,2,8–16). It is also classified as having low- or high-grade histology. Low-grade tumors include squamous cell carcinoma, mucoepidermoid carcinoma and basaloid carcinoma. High-grade tumors include lymphoepithelioma-like carcinoma, small cell carcinoma, poorly differentiated carcinoma, sarcomatoid carcinoma and clear cell carcinoma.
In general, it is thought that there is a close relationship between histological classification and prognosis of thymic carcinoma, although none has shown any prospective data to support this idea. Tumors in the low-grade histological group are characterized by relatively favorable clinical courses and a low incidence of local recurrence and distant metastasis, while tumors in the high-grade histological group are characterized by aggressive clinical courses and a high incidence of local recurrence and distant metastasis (2,10,12,13).
In our series, four patients had squamous cell carcinoma, which belongs to the low-grade histological group, and six had poorly differentiated carcinoma, which belongs to the high-grade histological group. Four of 10 patients responded to chemotherapy and the range of response duration was 5–13 months. This response rate is comparable to the results in previous reports and the duration of response was short irrespective of tumor histology.
In contrast to previous reports, however, the high-grade histological group had a longer MST than the low-grade histological group (13 versus 10 months, respectively), even though there was no statistically significant difference in survival between the two histological groups (p = 0.15). The reason for this discrepancy is not clear, but it is possible that platinum-based chemotherapy with or without thoracic radiation does not affect the prognosis of patients with unresectable thymic carcinoma.
Platinum-based chemotherapy may be utilized in advanced cases, although the clinical benefit of chemotherapy for unresectable thymic carcinoma is controversial. Reliable data on the prognosis of thymic carcinoma patients treated with chemotherapy are scarce (Table 4) (2–6), because thymic carcinoma is a very rare neoplasm. Most of these studies only retrospectively evaluated the effects of adjuvant or neoadjuvant chemotherapy in patients who had potentially resectable disease (2–5). However, Latz et al. (6) retrospectively described the prognosis of patients with unresectable thymic carcinoma who were treated with radiation therapy and chemotherapy, but were unable to give any valid recommendations about which chemotherapeutic regimen or dosage should be applied. In our series of patients with unresectable thymic carcinoma who were all treated by platinum-based chemotherapy with or without radiation, the results were comparable to those of Latz et al. Therefore, the effect of platinum-based chemotherapy on the survival of patients with unresectable thymic carcinoma remains to be determined and we cannot currently recommend it as a standard therapy for such patients.
In summary, we did not find any association between histological subtypes and prognosis in advanced thymic carcinoma patients who received chemotherapy with or without radiotherapy and showed that platinum-based chemotherapy is only marginally effective in advanced thymic carcinoma patients, resulting in a modest tumor response rate, short response duration and poor prognosis. More effective chemotherapy with new agents is required and, owing to the rarity of this tumor, multi-institutional trials are necessary for prospective evaluation of treatments.
For reprints and all correspondence: Yoichi Nakamura, Department of Internal Medicine, Nagasaki Municipal Medical Center, 20–5 Fuchi-machi, Nagasaki 852-8012, Japan. E-mail: nmmc2@bronze.ocn.ne.jp
Figure 1. Kaplan–Meier estimates of survival for all 10 patients assigned to histological groups. Thin line, low-grade histological group; thick line, high-grade histological group.
Clinical profiles of 10 patients with thymic carcinoma
| Case No. | Age (years) | Gender | Histology |
| Metastatic site | |||
| 1 | 44 | M | Poorly differentiated |
| 2 | 51 | M | Squamous cell |
| 3 | 44 | F | Poorly differentiated |
| Pleura | |||
| 4 | 27 | F | Poorly differentiated |
| Bone | |||
| 5 | 71 | F | Poorly differentiated |
| 6 | 50 | M | Poorly differentiated |
| Lung | |||
| 7 | 65 | M | Poorly differentiated |
| 8 | 53 | M | Squamous cell |
| Lung, pleura, bone | |||
| 9 | 65 | M | Squamous cell |
| Bone, eye, liver | |||
| 10 | 46 | F | Squamous cell |
| Case No. | Age (years) | Gender | Histology |
| Metastatic site | |||
| 1 | 44 | M | Poorly differentiated |
| 2 | 51 | M | Squamous cell |
| 3 | 44 | F | Poorly differentiated |
| Pleura | |||
| 4 | 27 | F | Poorly differentiated |
| Bone | |||
| 5 | 71 | F | Poorly differentiated |
| 6 | 50 | M | Poorly differentiated |
| Lung | |||
| 7 | 65 | M | Poorly differentiated |
| 8 | 53 | M | Squamous cell |
| Lung, pleura, bone | |||
| 9 | 65 | M | Squamous cell |
| Bone, eye, liver | |||
| 10 | 46 | F | Squamous cell |
Clinical profiles of 10 patients with thymic carcinoma
| Case No. | Age (years) | Gender | Histology |
| Metastatic site | |||
| 1 | 44 | M | Poorly differentiated |
| 2 | 51 | M | Squamous cell |
| 3 | 44 | F | Poorly differentiated |
| Pleura | |||
| 4 | 27 | F | Poorly differentiated |
| Bone | |||
| 5 | 71 | F | Poorly differentiated |
| 6 | 50 | M | Poorly differentiated |
| Lung | |||
| 7 | 65 | M | Poorly differentiated |
| 8 | 53 | M | Squamous cell |
| Lung, pleura, bone | |||
| 9 | 65 | M | Squamous cell |
| Bone, eye, liver | |||
| 10 | 46 | F | Squamous cell |
| Case No. | Age (years) | Gender | Histology |
| Metastatic site | |||
| 1 | 44 | M | Poorly differentiated |
| 2 | 51 | M | Squamous cell |
| 3 | 44 | F | Poorly differentiated |
| Pleura | |||
| 4 | 27 | F | Poorly differentiated |
| Bone | |||
| 5 | 71 | F | Poorly differentiated |
| 6 | 50 | M | Poorly differentiated |
| Lung | |||
| 7 | 65 | M | Poorly differentiated |
| 8 | 53 | M | Squamous cell |
| Lung, pleura, bone | |||
| 9 | 65 | M | Squamous cell |
| Bone, eye, liver | |||
| 10 | 46 | F | Squamous cell |
Treatment and response
| Case No. | Cx regimen | Courses | Response | RT (Gy) | Effect of RT |
| 1 | PACE | 4 | PR | – | |
| 2 | PACE | 2 | NC | 50 | NC |
| 3 | PACE | 4 | NC | 56 | NC |
| 4 | PACE | 1 | NC | 6 | NE |
| 5 | PACE | 4 | PR | 50 | NC |
| 6 | CBDCA + VP-16 | 2 | NC | 50 | PR |
| CAP | 2 | PR | |||
| PACE | 3 | NC | |||
| MVP | 3 | PR | |||
| 7 | PACE | 1 | NC | 50 | NC |
| 8 | CODE | (5 weeks) | PD | 50 | NC |
| 9 | CBDCA + VP-16* | 1 | PD | – | |
| 10 | MVP | 3 | PR | 50 | NC |
| Case No. | Cx regimen | Courses | Response | RT (Gy) | Effect of RT |
| 1 | PACE | 4 | PR | – | |
| 2 | PACE | 2 | NC | 50 | NC |
| 3 | PACE | 4 | NC | 56 | NC |
| 4 | PACE | 1 | NC | 6 | NE |
| 5 | PACE | 4 | PR | 50 | NC |
| 6 | CBDCA + VP-16 | 2 | NC | 50 | PR |
| CAP | 2 | PR | |||
| PACE | 3 | NC | |||
| MVP | 3 | PR | |||
| 7 | PACE | 1 | NC | 50 | NC |
| 8 | CODE | (5 weeks) | PD | 50 | NC |
| 9 | CBDCA + VP-16* | 1 | PD | – | |
| 10 | MVP | 3 | PR | 50 | NC |
Cx, chemotherapy; RT, radiation therapy; NE, not evaluated; PACE, cisplatin 60 mg/m2, cyclophosphamide 800 mg/m2, doxorubicin 45 mg/m2 and etoposide 80 mg/m2 for 3 days every 4 weeks; CBDCA + VP-16, carboplatin 300 mg/m2 and etoposide 120 mg/m2 for 3 days in every 4 weeks; CBDCA + VP-16*, carboplatin area under the treatment and response curve = 4 and etoposide 75 mg/m2 for 3 days; CAP, cyclophosphamide 800 mg/m2 and doxorubicin 50 mg/m2 on day 1, cisplatin 25 mg/m2 weekly in every 4 weeks; MVP, cisplatin 80 mg/m2 and mitomycin 8 mg/m2 on day 1, vindesine 3 mg/m2 on days 1 and 8 in every 4 weeks; CODE, weekly chemotherapy, cisplatin 25 mg/m2 weekly, vincristine 1 mg/m2 in weeks 1, 2, 4, 6 and 8, doxorubicin 40 mg/m2 and etoposide 80 mg/m2 for 3 days in weeks 1, 3, 5, 7 and 9.
Treatment and response
| Case No. | Cx regimen | Courses | Response | RT (Gy) | Effect of RT |
| 1 | PACE | 4 | PR | – | |
| 2 | PACE | 2 | NC | 50 | NC |
| 3 | PACE | 4 | NC | 56 | NC |
| 4 | PACE | 1 | NC | 6 | NE |
| 5 | PACE | 4 | PR | 50 | NC |
| 6 | CBDCA + VP-16 | 2 | NC | 50 | PR |
| CAP | 2 | PR | |||
| PACE | 3 | NC | |||
| MVP | 3 | PR | |||
| 7 | PACE | 1 | NC | 50 | NC |
| 8 | CODE | (5 weeks) | PD | 50 | NC |
| 9 | CBDCA + VP-16* | 1 | PD | – | |
| 10 | MVP | 3 | PR | 50 | NC |
| Case No. | Cx regimen | Courses | Response | RT (Gy) | Effect of RT |
| 1 | PACE | 4 | PR | – | |
| 2 | PACE | 2 | NC | 50 | NC |
| 3 | PACE | 4 | NC | 56 | NC |
| 4 | PACE | 1 | NC | 6 | NE |
| 5 | PACE | 4 | PR | 50 | NC |
| 6 | CBDCA + VP-16 | 2 | NC | 50 | PR |
| CAP | 2 | PR | |||
| PACE | 3 | NC | |||
| MVP | 3 | PR | |||
| 7 | PACE | 1 | NC | 50 | NC |
| 8 | CODE | (5 weeks) | PD | 50 | NC |
| 9 | CBDCA + VP-16* | 1 | PD | – | |
| 10 | MVP | 3 | PR | 50 | NC |
Cx, chemotherapy; RT, radiation therapy; NE, not evaluated; PACE, cisplatin 60 mg/m2, cyclophosphamide 800 mg/m2, doxorubicin 45 mg/m2 and etoposide 80 mg/m2 for 3 days every 4 weeks; CBDCA + VP-16, carboplatin 300 mg/m2 and etoposide 120 mg/m2 for 3 days in every 4 weeks; CBDCA + VP-16*, carboplatin area under the treatment and response curve = 4 and etoposide 75 mg/m2 for 3 days; CAP, cyclophosphamide 800 mg/m2 and doxorubicin 50 mg/m2 on day 1, cisplatin 25 mg/m2 weekly in every 4 weeks; MVP, cisplatin 80 mg/m2 and mitomycin 8 mg/m2 on day 1, vindesine 3 mg/m2 on days 1 and 8 in every 4 weeks; CODE, weekly chemotherapy, cisplatin 25 mg/m2 weekly, vincristine 1 mg/m2 in weeks 1, 2, 4, 6 and 8, doxorubicin 40 mg/m2 and etoposide 80 mg/m2 for 3 days in weeks 1, 3, 5, 7 and 9.
Survival (months), sites of locoregional recurrence and causes of death
| Case No. | Survival (months) | Recurrence site | Cause of death | |
| Progression free | Overall | |||
| 1 | 7 | 8 | Bone, neck lymph node | Tumor |
| 2 | 6 | 10 | Primary, bone, liver | Tumor |
| 3 | 9 | 16 | Primary, lung | Tumor |
| 4 | 2 | 13 | Primary, lung | Tumor |
| 5 | 5 | 20 | Primary, lung | Tumor |
| 6 | 13 | 34 | Primary, bone, lung | Tumor |
| 7 | 5 | 7 | Primary | Tumor |
| 8 | 8 | 11 | Bone, lung | Tumor |
| 9 | 1 | 6 | Primary, liver, lung | Tumor |
| 10 | 7 | 7 | None | Alive |
| Case No. | Survival (months) | Recurrence site | Cause of death | |
| Progression free | Overall | |||
| 1 | 7 | 8 | Bone, neck lymph node | Tumor |
| 2 | 6 | 10 | Primary, bone, liver | Tumor |
| 3 | 9 | 16 | Primary, lung | Tumor |
| 4 | 2 | 13 | Primary, lung | Tumor |
| 5 | 5 | 20 | Primary, lung | Tumor |
| 6 | 13 | 34 | Primary, bone, lung | Tumor |
| 7 | 5 | 7 | Primary | Tumor |
| 8 | 8 | 11 | Bone, lung | Tumor |
| 9 | 1 | 6 | Primary, liver, lung | Tumor |
| 10 | 7 | 7 | None | Alive |
Survival (months), sites of locoregional recurrence and causes of death
| Case No. | Survival (months) | Recurrence site | Cause of death | |
| Progression free | Overall | |||
| 1 | 7 | 8 | Bone, neck lymph node | Tumor |
| 2 | 6 | 10 | Primary, bone, liver | Tumor |
| 3 | 9 | 16 | Primary, lung | Tumor |
| 4 | 2 | 13 | Primary, lung | Tumor |
| 5 | 5 | 20 | Primary, lung | Tumor |
| 6 | 13 | 34 | Primary, bone, lung | Tumor |
| 7 | 5 | 7 | Primary | Tumor |
| 8 | 8 | 11 | Bone, lung | Tumor |
| 9 | 1 | 6 | Primary, liver, lung | Tumor |
| 10 | 7 | 7 | None | Alive |
| Case No. | Survival (months) | Recurrence site | Cause of death | |
| Progression free | Overall | |||
| 1 | 7 | 8 | Bone, neck lymph node | Tumor |
| 2 | 6 | 10 | Primary, bone, liver | Tumor |
| 3 | 9 | 16 | Primary, lung | Tumor |
| 4 | 2 | 13 | Primary, lung | Tumor |
| 5 | 5 | 20 | Primary, lung | Tumor |
| 6 | 13 | 34 | Primary, bone, lung | Tumor |
| 7 | 5 | 7 | Primary | Tumor |
| 8 | 8 | 11 | Bone, lung | Tumor |
| 9 | 1 | 6 | Primary, liver, lung | Tumor |
| 10 | 7 | 7 | None | Alive |
Reports ofchemotherapy for thymic carcinoma
| Reference | Cx/N | Regimen | (CR + PR)/N | MST or survival |
| 2 | 26/60 | NR | NR | 1 year 56.6% |
| 3 | 8/8 | ADR, CAV(P) | 2/8 | MST 70.0 months |
| 4 | 12/20 | Cisplatin-based | NR | MST 39.0 months |
| MST* 14.3 months | ||||
| 5 | 5/5 | Cisplatin-based | 3/5 | NR |
| 6 | 9/10 | NR | NR | MST 12.0 months |
| This study | 10/10 | Cisplatin-based | 4/10 | MST 10.0 months |
| Reference | Cx/N | Regimen | (CR + PR)/N | MST or survival |
| 2 | 26/60 | NR | NR | 1 year 56.6% |
| 3 | 8/8 | ADR, CAV(P) | 2/8 | MST 70.0 months |
| 4 | 12/20 | Cisplatin-based | NR | MST 39.0 months |
| MST* 14.3 months | ||||
| 5 | 5/5 | Cisplatin-based | 3/5 | NR |
| 6 | 9/10 | NR | NR | MST 12.0 months |
| This study | 10/10 | Cisplatin-based | 4/10 | MST 10.0 months |
Cx, number of patients who received chemotherapy; N, total number of patients; NR, not reported; ADR, doxorubicin; CAV(P), cyclophosphamide, doxorubicin and vincristine (cisplatin); MST, median survival time; MST*, MST for patients who were incompletely resected.
Reports ofchemotherapy for thymic carcinoma
| Reference | Cx/N | Regimen | (CR + PR)/N | MST or survival |
| 2 | 26/60 | NR | NR | 1 year 56.6% |
| 3 | 8/8 | ADR, CAV(P) | 2/8 | MST 70.0 months |
| 4 | 12/20 | Cisplatin-based | NR | MST 39.0 months |
| MST* 14.3 months | ||||
| 5 | 5/5 | Cisplatin-based | 3/5 | NR |
| 6 | 9/10 | NR | NR | MST 12.0 months |
| This study | 10/10 | Cisplatin-based | 4/10 | MST 10.0 months |
| Reference | Cx/N | Regimen | (CR + PR)/N | MST or survival |
| 2 | 26/60 | NR | NR | 1 year 56.6% |
| 3 | 8/8 | ADR, CAV(P) | 2/8 | MST 70.0 months |
| 4 | 12/20 | Cisplatin-based | NR | MST 39.0 months |
| MST* 14.3 months | ||||
| 5 | 5/5 | Cisplatin-based | 3/5 | NR |
| 6 | 9/10 | NR | NR | MST 12.0 months |
| This study | 10/10 | Cisplatin-based | 4/10 | MST 10.0 months |
Cx, number of patients who received chemotherapy; N, total number of patients; NR, not reported; ADR, doxorubicin; CAV(P), cyclophosphamide, doxorubicin and vincristine (cisplatin); MST, median survival time; MST*, MST for patients who were incompletely resected.
References
1 Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts.
2 Suster S, Rosai J. Thymic carcinoma. A clinicopathological study of 60 cases.
3 Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M. Treatment and prognosis of primary thymic carcinoma.
4 Hsu CP, Chen CY, Chen CL, Lin CT, Hus NY, Wang JH, et al. Thymic carcinoma. Ten years’ experience in twenty patients.
5 Weide LG, Ulbright TM, Loehrer PJ, Williams SD. Thymic carcinoma. A distinct clinical entity responsive to chemotherapy.
6 Latz D, Schraube P, Oppitz U, Kugler C, Manegold C, Flentje M, et al. Invasive thymoma: treatment with postoperative radiation therapy.
7 Oshita F, Ohe Y, Tamura T, Eguchi K, Shinkai T, Saijo N, et al. Intensive chemotherapy with cisplatin, doxorubicin, cyclophosphamide, etoposide and granulocyte colony-stimulating factor for advanced thymoma or thymic cancer: preliminary results.
8 Tsuchiya R, Koga K, Matsuno Y, Mukai K, Shimosato Y. Thymic carcinoma: proposal for pathological TNM and staging.
9 Snover DC, Levine GD, Rosai J. Thymic carcinoma. Five distinctive histological variants.
10 Truong LD, Mody DR, Cagle PT, Jackson-York GL, Schwartz MR, Wheeler TM. Thymic carcinoma. A clinicopathological study of 13 cases.
11 Wick MR, Scheithauer BW, Weiland LH, Bernatz PE. Primary thymic carcinomas.
12 Shimosato Y, Kameya T, Nagai K, Suematsu K. Squamous cell carcinoma of the thymus. An analysis of eight cases.
13 Kuo TT, Chang JP, Lin FJ, Wu WC, Chang CH. Thymic carcinomas: histopathological varieties and immunohistochemical study.
14 Matsuno Y, Mukai K, Noguchi M, Sato Y, Shimosato Y. Histochemical and immunohistochemical evidence of glandular differentiation in thymic carcinoma.
15 Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcimoma: a clinicopathological study of 22 cases.
Author notes
1Thoracic Oncology Division and 3Radiation Oncology Division, National Cancer Center Hospital, Tokyo and 2Second Department of Internal Medicine, Nagasaki University School of Medicine, Nagasaki, Japan+