Well Before It Might Produce Hearts And Livers, 3D Bioprinting Is Reshaping Modern Medicine

Ibrahim Ozbolat loves his work so much that once he nearly sacrificed a finger for its sake. It was in 2007 or 2008, he recalled, when he was a student who had spent many hours straight in tunnel-like concentration on the 3D bioprinter before him.
Suddenly, his hand felt like it was being squeezed into a part of the machine. “I was about to lose my finger,” Ozbolat remembered. He rushed to the doctor just in time, and a decade later, only a thin scar remains. Ozbolat, now an associate professor at the Huck Institutes of the Life Sciences at Pennsylvania State University, wears it like a badge of honor.
“This is dedication,” he said.
Today, Ozbolat is in good company. Many others are staking a great deal, though perhaps not their extremities, on 3D bioprinting. Drawn by the promise of saving lives, healing people and tapping into the lucrative world of pharmaceuticals and medicine, academic researchers and biotech companies alike are flocking to this niche field, trying to usher it along a path toward the holy grail of printing solid organs like hearts or livers. Yet even their discoveries along the way are beginning to reshape and push the limits of modern medicine.
“There has been very large growth in terms of the applications that are being investigated, the printing systems that are available, as well as advances in scientific understanding,” said Dr. Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine in Winston-Salem, North Carolina. Scientists can build models for surgery — one of the least complex processes, Atala said — or build tiny organoids of human cells that can be used for screening drugs. And that’s just the beginning.
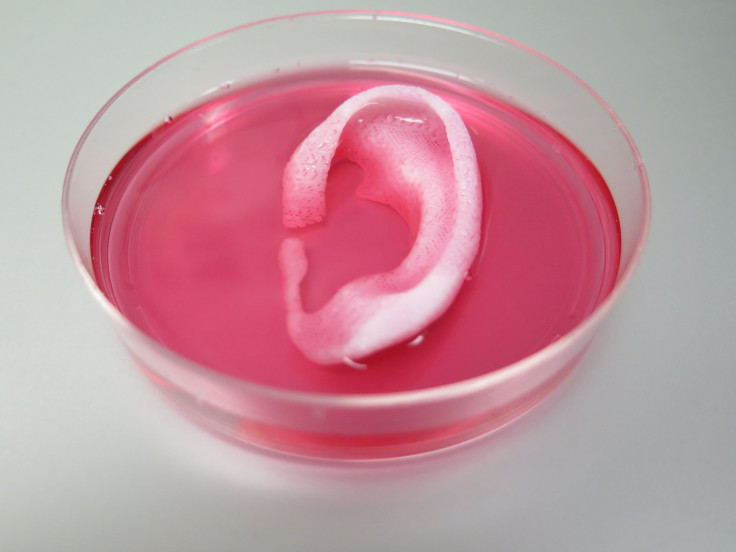
In 3D bioprinting, live cells are laid out in such a way that they can become bone, cartilage or other tissue in the human body that can be used in medicine and research. Because the cells are alive, the process is vastly more complicated than the already complex art of 3D printing. After they are printed, the cells must also be programmed or manipulated to perform a specific task. They have to be kept alive, too.
Creating live tissue outside the human body is not a new practice. Even before high-tech bioprinters were developed, doctors and researchers were able to engineer living tissues and some organs. They just had to do it manually, in a laborious process that wasn’t as precise or easy to replicate as researchers wished.
The advent of 3D bioprinting in the early 2000s changed all that.
“We can create the same tissue in the same manner every time,” said Atala, who led the team that in 2006 reported the first successful transplant of a bladder manually engineered in a laboratory. With 3D bioprinting technology, engineering tissues like skin, bone and cartilage can become more precise, more scalable, more reproducible.
The technology is valuable and promising enough to have garnered shoutouts and financial support from the government. Atala estimated that he had received tens of millions of dollars in federal funding, including from the National Institutes of Health and the Department of Defense, to work on 3D bioprinting, and that such funding had increased in recent years.
In November, researchers at Carnegie Mellon University funded by the NIH developed a unique 3D printer. It was more affordable than other systems, NIH director Dr. Francis Collins wrote in a blog post, and the unique scaffolds it could print would “set the stage for the creation of custom-made tissues and organs with unprecedented anatomical detail.”
The NIH is funding other 3D bioprinting projects, too. This year, according to a database of NIH awards, it granted $135,700 to Binata Joddar, a bioengineer at the University of Texas at El Paso, for a project in bioprinting a type of stem cell in order to “form engineered cardiac patches.”
The question that tends to dominate 3D bioprinting — when full-fledged organs will be available — sometimes masks the strides that scientists have already made in the field.
It takes about 25 to 30 minutes to print the trachea, made of cartilage, that a team of researchers at the Feinstein Institute for Medical Research in Manhasset, New York, is currently working on. One of the investigators, Todd Goldstein, pointed out that the entire process — planning the printing beforehand and culturing the cells afterward — takes about two weeks.
“We actually take the patient’s own cells and then print the actual trachea,” Goldstein said. “It’s living, breathing, doing all the cellular metabolic activities.” Currently, 3D bioprinted windpipes cannot be planted in humans, but it’s these kinds of experiments that are paving the way toward more ambitious work and sea changes in some of the most basic concepts in medicine.
To fix a car, for instance, a mechanic can either repair a broken part or order a new one. “For physicians, they don’t have that option of getting a new part,” Goldstein explained. Bioprinting has the potential to change that, albeit in small steps. “Certain pieces of anatomy will come first,” like skin, bone and cartilage, he added.
Solving the challenges in building those simpler tissues will be essential to creating more complex ones. For Goldstein and his team, getting cells to work together the way they would in the human body is one of the biggest hurdles.
“We have a lot of hype in this field...Printing cells doesn’t mean you make a tissue or an organ,” he said.
“If a year down the line you have uncontrolled growth then you just gave someone cancer,” he said. The reverse, where the cells fail to grow or they die, means a person now has a hole in their windpipe. “You want to make sure the tissue looks, acts and feels like it’s supposed to,” Goldstein said.
As the bioprinting possibilities expand, they are luring companies to the 3D bioprinting sector and fueling predictions of a coming boom. In a report released in November, Grand View Research said the market for 3D bioprinting would grow to $1.82 billion by 2022 from $487 million in 2014. The industry for bioprinters is projected to mirror that growth, from $562.8 million in 2015 to $1.9 billion in 2022, according to predictions by Credence Research.
Leading the pack are companies like Organovo, which is working on developing liver tissue, and in May 2015 signed a deal with L’Oreal to provide the French cosmetics company with skin for testing products. In November, a laboratory in Moscow claimed to have printed a thyroid and successfully transplanted it into a mouse.
Those don’t include the companies that are making printers themselves, like MicroFab Technologies Inc., which partnered with the Wake Forest Institute of Regenerative Medicine to develop a skin bioprinter , or the German company EnvisionTEC’s $188,000 printer, dubbed the Bioplotter. The Philadelphia-based company BioBots, which makes the $10,000 desktop Biobot 1, has continually wowed visitors at exhibitions with its miniature printed Van Gogh ear.
In the face of so much excitement, though, scientific hurdles and regulatory questions remain, and government agencies have not yet decided how to treat inventions that not so long ago seemed the stuff of science fiction.
“Devices constructed using 3D printing technology are subject to the same regulatory review standards as devices constructed using traditional manufacturing practices,” said Deborah Kotz, a spokesperson for the federal Food and Drug Administration. The agency does not have definite dates for when it will update that guidance, although “as 3D printing technology develops, FDA may provide further clarity on quality control issues such as material qualifications and sterility,” Kotz added.
“We have a lot of hype in this field,” said Ozbolat, the Penn State professor who nearly gave a finger to his bioprinter. He now studies vascularization, or the formation of veins and arteries, and said that progress in the field in recent years had been “huge” but still has a long way to go. “Printing cells doesn’t mean you make a tissue or an organ,” he said.
Another unsolved problem is how to keep cells alive after they’re printed, although some are hardier than others.
“Bone, skin, cartilage cells are strong and resilient,” Ozbolat said. Cancer cells simply refused to die, he added, while pancreatic cells gave up all too easily. “You treat them like your baby, but they die … because they’re very weak,” he said.
The future of 3D bioprinting depends on solving these kinds of problems, Ozbolat said. “If you don’t really make progress in all of this, bioprinting will not be a useful technology. Something cool, but not really functional.”
© Copyright IBTimes 2024. All rights reserved.












