Nanotechnology-based food and health products and food packaging materials are available to consumers in some countries already, and additional products and applications are currently in the research and development stage, and some may reach the market soon. In view of such progress, it is expected that nanotechnology-derived food products will be increasingly available to consumers worldwide in the coming years.1
World Health Organization/United Nations Food and Agriculture joint expert report, 2010
Yes, we have no nano-coated bananas, no bananas today
According to the WHO/FAO expert panel, it is “progress” that agri-nanotechnology products are on the market and that more products are on their way to commercialization. WHO and FAO are also the parent organizations of the Codex Alimentarius Commission, the international food standards setting body. Codex has yet to agree on any agri-nanotechnology standards, nor indeed, even to begin work on such standards to protect consumer health, even though that is a core part of its mandate. In the meantime, is it “progress” that agri-nanotechnology products are in the marketplace without any regulation?
Let us consider the humble banana, by volume, it’s the most exported fruit in the world.2 Would you eat a banana whose peel had been coated with a mixture of Engineered Nanoscale Materials (ENMs), designed to retain moisture and retard spoilage better than can be done with conventional food coatings? Would you eat such a banana when there had been no pre-market safety assessment to determine whether ENMs would pass through the peel to the flesh of the banana? (ENMs are defined by their size shape and properties. Definitional debates are discussed below, but comparative sizes give an idea of how small ENMS are, e.g., if a nanoparticle were a football, a doughnut would be the size of New Zealand.3 Insofar as the coating is transparent and there is no labeling of such produce, you wouldn’t even know, much less have a choice. Unfortunately, these questions aren’t hypothetical.
A U.S. Department of Agriculture official cited anonymously by Pulitzer Prize winning journalist Andrew Schneider suggested that nano-coated produce is already being shipped from Latin America. The Food and Drug Administration (FDA), the agency charged with regulating produce and imported food, denies that there are any foods with ENMs in U.S. commerce.4
The manipulation of atomic-to molecular-sized nanoparticles (NPs) has many commercially attractive properties for manufacturers of consumer and industrial products. For example, more than a decade of research on the incorporation of ENMs into packaging for food has identified a number of applications to extend the shelf-life of packaged foods, and even detect contamination of packaged food.5 Food nanocoatings are just one of several food packaging applications of nanotechnology in research and development.6 As of 2008, about 50 kinds of biopolymers had been electronically spun into nanofibers that can be woven into “mats” with properties of great interest to the food packaging and processing industries.7 Nanofibers will, in theory, prevent other ENMs from passing through the biopolymer to penetrate the packaged food. Food processors believe that coating food production belts with nano-silicon dioxide will prevent bacteria from adhering to food production machinery, reducing the likelihood of food contamination and the cost of cleaning the machinery.8
Both organic and inorganic nano-sized additives, such as silver, platinum, silicon and iron, are incorporated into food packaging, food and feed, according to an industry overview. Although the survey characterizes which applications are in a research and development stage and which have been commercialized, the survey is generic, and does not name companies or products.9 A Friends of the Earth Australia report, based on industry product claims, identifies product names, companies and web addresses for the product claims of a broad array of ENMs in food packaging, food contact surfaces, food ingredients and in foods, all on the market place without regulation.10 Given official denials11 that no nano-coated produce has been authorized for import into the United States, we can confidently assume, to invoke the old Broadway song, that “yes, we have no nano-coated bananas, no bananas today.”
However, some of these products appear to already be in commercial use in other countries, raising the likelihood that they could become a part of our food supply in the near future, if they are not already. The global food and food packaging business offers a potentially huge market for ENMs. For the Grocery Manufacturers Association, the largest association of food retailers, which claims aggregate sales of $2.1 trillion annually, the projected market for nano-enabled packaging materials in the next decade could amount to 25 percent of the total $100 billion annual food packaging market.12
Whether or how these nanocoatings might migrate off the packages and onto food or into human bodies, however, is not yet known. There is little gastro-intestinal risk assessment, particularly for chronic exposure to ENMS, to serve as a basis for standard setting. For example, a 2012 U.S. National Research Council report states, ”little research progress has been made on some key topics, such as the effects of ingested ENMs on human health.”13 Given this lack of research, there aren’t any food safety standards that would enable exporters to trade such produce internationally, at least not within a legal framework. Will such standards be agreed, and if so, when, and with what content? Furthermore, how would they be implemented and enforced?
Regulators and the pressure to commercialize ENMs
Despite the data gaps and methodological uncertainties that limit risk assessment of ENMs, researchers and regulators of the environmental health and safety effects of nanotechnologies are under great pressure to also be entrepreneurs for the technologies. On June 9, 2011, the Environmental Protection Agency (EPA) published a request for comment on draft voluntary guidance for submitting data on ENMs in pesticide products to the EPA.14 IATP was one of the petitioners that had demanded in 2008 that EPA require submission of nano-silver data in EPA approved and yet to be approved pesticide products. 15 That same day, the White House released a memorandum citing the following justification for informing agency heads about how they are to regulate ENMs and products incorporating ENMs.
Our regulatory system must protect public health, welfare, safety and the environment, while promoting economic growth, competitive, innovation and job creation. It must be based on the best available science.
President Barack Obama, Executive Order 13563, January 18, 2011, cited in the June 9 White House memo
The White House memo was mentioned on June 9 in the Food and Drug Administration’s general request for comment on how the agency should determine if there are ENMs in FDA-regulated products.16 The net effect of the memo is to remind agencies heads with statutory EHS mandates that they are also to ensure that the regulatory system is an adjunct to trade and job creation. The memo doesn’t advise the agency’s head what to do if the best available science shows that an ENM or a nano-enabled product presents risks too prevalent and severe to allow for its commercialization. On December 21, 2011, IATP joined five other NGOs, led by the International Center for Technology Assessment, in suing the FDA for failing to respond to a May 2006 petition to regulate ENMs, particularly nano-titanium dioxide.17
Whatever the FDA response to this lawsuit, the agency will be under White House pressure to carry out its statutorily authorized responsibilities for food safety in the context of the pressure also to be technology promoters. As is detailed in the following section, U.S. regulators are subject to carrying out their environmental health and safety protection duties under World Trade Organization commitments to ensure that food safety rules are least trade restrictive and not a “disguised barrier to trade.”
When the European Parliament issued a non-binding resolution calling for the mandatory labeling of products with ENMs, the American Chemical Council called on the Office of the U.S. Trade Representative, a co-author of the June 9 memo, to threaten the European Commission with a trade dispute to prevent the approval of any labeling legislation. Such trade dispute threats could be in the near future of some applications of agri-nanotechnology.18
The trade policy context of food safety standards
How might the Codex Alimentarius Commission set standards for an ingredient of a nano-coating compound deemed by Codex to be a food additive? Standards are numerically defined, such as Average Daily Intakes for additives or Maximum Residue Levels for pesticides or veterinary drugs. Just a quick scan of the “List of Standards” shows the thousands of food, food ingredient, livestock drug and pesticides that have been standardized.19 Codex writes standards about generic additives in isolation, not standards about the combination and interaction of additives in specific products. The Commission could decide to negotiate not just numerical standards on nanoscale food additives but also Codes of Practice, Guidelines or Principles for the use of ENMs in food packaging and food nano-coatings.
Codex negotiates standards to implement the Joint FAO/WHO Food Standards Program, “the purpose of which is protecting the health of the consumers and ensuring fair practices in the food trade.”20 Codex standards, negotiated by delegates from over 180 member governments and more than 110 international nongovernmental organizations, are presumed to be authoritative in the World Trade Organization Agreement on Trade Related Sanitary and Phytosanitary Measures (SPS Agreement, Article 3.3). The SPS Agreement enjoins WTO members to participate in Codex meetings “within the limits of their resources” (Art. 3.4).
Since the WTO’s founding in 1995, the protection of consumer health by Codex standards has been subject to the SPS Agreement’s injunction that governments justify that each and every SPS measure does not constitute “a disguised restriction on international trade” (Article 3.5). The SPS Committee members may request clarifications about national SPS rules that one or more members believes to violate the SPS Agreement. If the clarifications are unsatisfactory, one member may file a trade dispute complaint to try to force the other member to change the offending rule. There is a long list of WTO member trade disputes that cite violations of the SPS Agreement.21
To regulate according to standards more stringent in the protection of human health than those of Codex, WTO members must prove their food safety measures are “least trade restrictive” and “necessary” to fulfill a regulatory objective pursuant to the WTO member’s “appropriate level of sanitary or phytosanitary protection” (Art. 5.6). In determining food safety rules and other SPS measures, WTO members are to “take into account” all relevant factors, including the exceptional character of human health risks to which people voluntarily expose themselves” (Article 5.5).
The WTO SPS Committee has developed guidelines to ensure consistency among WTO members in application of this particularly controversial article.22 However, these guidelines do not clarify how WTO members are supposed take into account the risks assumed by consumers voluntarily. 23 Labeling of food and food ingredients is one way to enable consumers to take on informed risks, e.g., to consuming transfats.
Codex recognizes that “food labeling plays an important role in furthering both of these objectives” [the protection of consumer health and ensuring fair practices in the trade of food].24 However, Codex battles on food labeling guidance can be long and intense, 20 years long in the case of labeling for genetically modified foods.25 In the United States, continuing opposition to GM food labeling has been justified as protecting a U.S. Constitutional right to free commercial speech,26 implying that any U.S. GM labeling law could prompt a case before the U.S. Supreme Court. IATP is a signatory to “Principles for the Oversight of Nanotechnologies and Nanomaterials,” according to which, ”The public’s right to know requires the labeling of all products containing nanomaterial ingredients.”27 To judge by the aforementioned request to launch a WTO dispute against EU labeling of products with ENMs, mandatory labeling of foods with ENMs would face opposition similar to that advocated by proponents of GM foods.
In summary, trade according to WTO rules, particularly those of the SPS Agreement, has come to dominate much of the Codex debate about food standards. Anticipated loss of trade will very likely affect the Codex debate on standards for ENMs in food additives. A Codex provision allows members to opt out of Codex food additive standards by stating that additives within the General Standard for Food Additives are “subject to the national legislation of the importing country.” This provision has been used chiefly for coloring and sweetening additives. The United States, together with several African and Latin American countries sought deletion of the provision at the Codex Committee on Food Additives meeting in March in Beijing.28 These Codex member governments are concerned that the provision can be used to impede trade and that the opt-out provision undermines the science-based principle of Codex standards. As we detail below, standards for food nanocoatings likely would be first negotiated in the Committee on Food Additives.
Nanotechnology at Codex thus far
In July 2011, at the annual meeting of the Codex Alimentarius Commission, Egypt requested that Codex establish a task force on food and agricultural applications of nanotechnology. Because most Codex work is first done through committees, the request for a task force signified that Egypt believed the application of nanotechnology to food raised too many challenges to be for standards work to be assigned to one or two committees. Egypt had made the same request in 2010, noting that dietary supplements and food additives with ENMs have been traded internationally without regulation.
According to the meeting report, FAO and WHO officials offered a dual response to Egypt. First, WHO and FAO had convened an expert meeting on nanotechnology applications in June 2009. Among the recommendations and conclusions in the meeting report, this one stood out because of its relationship to Codex’s standard-setting work: “The current risk assessment approach used by FAO/WHO and Codex is suitable for Engineered Nano-scale Materials in food and agriculture, including the effects of ENMs on animal health.”29
This conclusion might lead one to think that Codex would request FAO and WHO to convene one or more risk assessment panels to begin the standards setting process for those ENMs known to be incorporated in food and feed products traded internationally. For example, Codex could request the Joint FAO/WHO Expert Committee on Food Additives (JECFA) to carry out risk assessments of generic ingredients reportedly used in nano-enabled food packaging, such as nano-silicon dioxide (to prevent clumping of the coating), nano-titanium dioxide (to prevent damage by ultraviolet rays), nano-silver (a biocide) and nano-clays (to prevent moisture loss). Or it could review the scientific literature on those food nanocoatings that claim to be edible, to determine whether nano-sizing of “food-grade” ingredients, such as fuma silica, a thickening agent, results in properties that pose risks to human health.30 On the basis of the resulting risk assessments, Codex would elaborate standards for each nanocoating ingredient. Then we could remove the question mark from this paper’s title.
More broadly, the beginning of standard-setting at the very least would reduce the perception that commercialization of consumer products with ENMs will continue under a de facto industry self-regulation of ENMs. Self-regulatory failure in agri-nanotechnology may result in consumer rejection not just of any one application but of nanotechnology in general. A U.S. Food and Drug Administration (FDA) official advised the industry to also address consumer concerns about applications of nanotechnology to food in terms not directly related to the science reviewed by risk assessors, what Codex terms “other legitimate concerns.” Otherwise, the official advised, nanotechnology promoters could face a commercial backlash similar to that experienced by genetically modified organisms.31
Rather than proceed with the establishment of a task force, however, FAO and WHO officials told Egypt that Codex should wait for “other entities to complete their work” on nanotechnology regulation before Codex begins its work. Nanotechnology standards from the United States and European Commission, as well as from the Organization for Economic Cooperation and Development (OECD), apparently are to guide to Codex agri-nanotechnology work. However, this work of “other entities” is proceeding very slowly and is far from completion.32
Lack of clarity on the definition of “nanomaterials”
One of the impediments to regulation has been lack of agreement about a definition of “nanomaterials” that would be applicable by regulators and yet flexible enough to encompass the fast developing science about ENMs. In October 2011, after more than two years of debate, the European Commission (one of the “other entities”) published its recommendation for a definition of a “nanomaterial” for regulatory purposes.33 The definition references the scientific literature concerning the size range, shapes and unique properties of nanomaterials.
The definition includes a size range of one to 100 nanometers (nm). The Transatlantic Consumer Dialogue, of which IATP is a member, had recommended a one to at least a 300 nanometer range, with a 1000 nm limit for drugs with nanoparticles. The higher recommended limit reflects scientific research in which larger ENMs of 240 nm can breach the human placenta barrier.34
However, at the insistence of the huge industry lobby in Brussels and the EC’s Directorate General of Enterprise, aspects of the definition and its application may be modified for reasons of trade “competitiveness.” Therefore, the regulator has the discretion to choose elements from the definition that would give an EU product with ENMs a competitive advantage in trade. In addition to defining nanoparticles’ size, the European Environmental Bureau had recommended a one-percent threshold of ENMs in a material to qualify as a nanomaterial.35 However, according to the Commission definition, “In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1 and 50%.” The Commission’s definition apparently tries to straddle the one percent threshold called for by some scientists and NGO advocates and the 50 percent threshold demanded by industry. Under the recommendation then, a material could be regulated as a nanomaterial if it contained as little as one percent to as much as 50 percent of nanomaterial content. But the definition is further qualified in a way that makes it unclear what would be regulated.
The industry also lobbied successfully to include in the definition naturally occurring nanomaterials, e.g., smoke particles, as well as “incidental” nanomaterials produced during manufacture, rather than only engineered nanomaterials, the original subject of the definition. Critics of the definition believe that the changes to the draft definition will reduce regulators’ ability to protect consumers and the environment from products that incorporate ENMs, since the regulators would also have to estimate what part of the product content concerned “natural” and “incidental” nanomaterials versus that part incorporating ENMs. The recommended definition must be approved by the European Parliament and Council for use in legislation and rulemaking. Once approved the definition must be reviewed, in light of new scientific information, by December 2014. (Nanotechnology is among the industries covered by the Corporate Europe Observatory’s “Revolving Door Watch” project.36)
Notwithstanding intensive industry lobbying on the definition of “nanomaterials,” Mike Knowles, a Coca-Cola executive, assured participants at the Fourth Nanotechnology Stakeholder Day in 2011 in Brussels that food companies are not rushing to add ENMs to foods and food packaging materials. According to Knowles, “we’ve been living with nanostructures forever,” such as those naturally occurring nanomaterials in fats and carbohydrates. He told attending journalists that the “European regulatory system has all the safety aspects covered” but that European “technology aversion” was delaying introduction there of products with nanomaterials.37
Agreement on the definition of “nanomaterial” is clearly a critical first step in regulation. However, there is no need for Codex to continue to wait for “other entities” to decide how to regulate nanotechnology. The process to develop agri-nanotechnology standards and other related guidance texts, which will surely take several years, could begin now, incorporating findings from the other entities’ regulatory processes along the way.
The Codex standards setting process in theory and practice
As indicated in the aforementioned FAO/WHO expert report, Codex will likely develop agri-nanotechnology standards within its present standards setting process. That process is outlined here specifically for setting food-additive standards. Then risk assessment challenges of nano-silicon dioxide will be used to illustrate some of the difficulties that agri-nanotechnological standards may face in a trade policy context. Finally we will consider, from the viewpoint of food safety, both the advantages and disadvantages of Codex agri-nanotechnology standards.
The Codex standards setting process, as diagrammed in Understanding Codex (2006), seems straight forward enough.38 Since the founding of Codex in 1961, a great deal of effort has been devoted to developing a uniform standards setting process to enable Codex members to agree on a very large corpus of guidance texts, codes of practice and numerically expressed standards. Among the standards is the online data base of the General Standard for Food Additives, which lists over 1,500 standards, including those for macro-scale silicon dioxide and titanium dioxide. (According to a recent Pew Foundation report, additives are about half of the 10,787 substances that the FDA allows in food.39)
In practice though, the process is not quite so straight-forward. The first hurdle is to gather a critical mass of Codex members to launch a proposal for a standard. In some cases, one company, such as Monsanto, demanding that one member, such as the United States, propose a standard, such as recombinant Bovine Growth Hormone, suffices to start work. More often, a number of countries with trade interests in a product affected by a standard will combine to propose a standard setting project document.
Typically the Commission assigns work on a standard to a committee, which reports the status of its work to the Commission and to other committees that request such reporting. Work on the ingredients of a food nanocoating probably would be assigned to the Committee on Food Additives, a general subject committee.40 Other committees could be requested by the Commission to take up work on some aspect of agri-nanotechnology standard setting.
For example, since the mass of nanoparticles is not very relevant to dose metrics to measure toxicological exposure, the Committee on Methods of Sampling and Analysis (CCMAS) might want to propose a standard on particle size distribution for an ENM, rather than try to adjust a mass based standard originally developed for the macro-sized counterpart of the ENM. The Committee could then consider how to develop guidance on nano-particle number and surface area to arrive at a dose exposure for an ENM. The Codex Committee on Food Labeling might proposed a labeling guidance text for ENMs, once the Committee on Food Additives had agreed on a standard or even while the standard was in process.
For the sake of argument, let’s assume that Codex does not approve a task force on agri-nanotechnology, but instead initiates work through the Committee on Food Additives, hosted by China. How would the Committee proceed with its work?
The Executive Committee that reviews standards project proposals (their meetings are now audio-archived on the new Codex website) ensures that the proposals conform to terms in the Codex Procedural Manual, such as the definition of a food additive, and the pre-ambular requirements of the General Standards for Food Additives.41
A decision-tree diagram from the General Standards for Food Additives shows how the Committee on Food Additives would approach the first two steps of the general eight step Codex standards elaboration procedure. First, the Joint FAO/WHO Expert Committee on Food Additives (JECFA), must give a positive risk assessment of the additive for its intended use, in order for standard-setting to go forward. JECFA risk assessments can be contentious, particularly if many of the scientific studies reviewed for the risk assessment are industry studies. Second, the discussion on whether there is a technical justification for advancing a standard can be contentious, particularly if the risk assessment is controversial.
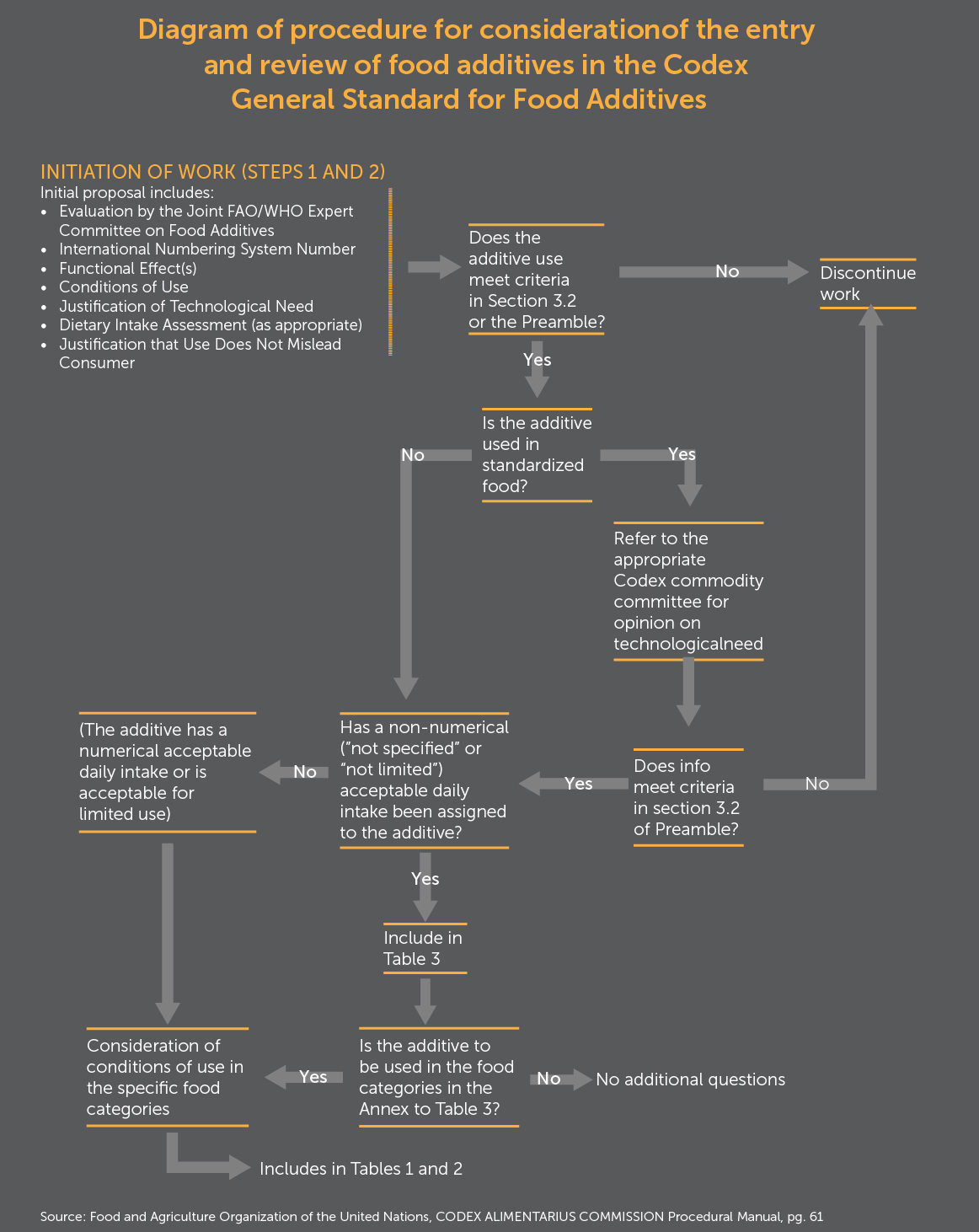
In the case of non-controversial standards, agreement on a draft standard in the committee can go straight to the Commission for approval at Step 8 of the eight-step process. In the case of very controversial standards, such as for the livestock growth hormones ractopamine or recombinant Bovine Growth Hormone, prolonged disagreement about the scientific basis for a standard, but refusal by its proponent to discontinue work on the standard, can result in its being “parked” for years at Step 8, without approval. Although standards are generally approved by consensus, voting is allowed in cases of highly controversial standards.42
If an ENM is not added to a food or feed, but, for example, is used to coat the surface of a food processing machine, Codex may decide that there is no need for expert risk assessment on which to base a numerically defined standard. Instead a guideline could be written about nanocoating food processing machinery that would incorporate a testing protocol about ENM residues on the processed food. Such a guideline is likely to be less controversial than the development of a standard for a nano-scaled food additive.
If, however, risk assessments about the ENM additives in coatings for fruits and vegetables are highly disputed and/or not transparent within JECFA, it would not be surprising to see at least the first proposals for ENM food additive standards to land in Step 8. Such controversies, if of sufficient commercial importance to the proponents of the standard, could also result in the launching of a trade dispute at the WTO.
A sample challenge to standard setting: risk assessing nano-silica
Let’s consider challenges to risk assessment posed by one likely component of a food nanocoating, nano-silicon dioxide. In “Presence and risks of nanosilica in food products” by Susan Dekkers et al., schematizes risk assessment factors.43 The authors believe that “the present study is the first to describe all the steps of the risk assessment process for the use of one specific nanomaterial (nano-silica particles) in food products.” They also note that the lack of peer-reviewed studies on the effects of specific nanomaterials in the gastro-intestinal tract has reduced greatly the relevance of general schemata for this risk assessment.
Their study estimates the presence and intake of nanosilica in 27 food products that are labeled as containing a non-nano synthetic amorphous silica. The percentage of nano-silica relative to all silica concentrations in these foods ranged widely, from 33 percent in an instant asparagus soup to four percent in a steak house rub. Estimating the human intake of nanosilica via food involves development of a laboratory simulacrum of human digestion for average concentrations and portion sizes of specific food products with nano-silica.44 The researchers were able to estimate exposure assessment, albeit within the limitations of stated assumptions about daily portion sizes and frequency of consumption.
However, because the “mechanism of silicon absorption from the gastro-intestinal tract has not yet been clarified,” the researchers could not determine whether the exposure to nanosilica would be hazardous to human health, in the event that nanosilica bio-accumulates in the human body, i.e. the second risk assessment scenario. If nanosilica dissolves and is excreted as non-nano silica, then no harm to human health from consuming nanosilica in food is anticipated.
The researchers concluded on the basis of 10-week long oral toxicity studies with rats and mice that “nanosilica becomes bioavailable to some degree and can exert toxicity on the liver. It is not clear how the silica is absorbed and if these effects are caused by nanosilica particles, dissolved silica or a combination of these two.”45 Absent a clear understanding of how nano-silica is absorbed, the toxicity of non-nano and nanosilica in a food product is difficult to compare. Without going further into the details of their research, suffice it to say that this difficulty and others would have to be reported in a critical mass of peer-reviewed studies in order for JECFA to agree on a risk assessment that would be a recommendation to Codex for a nano-silica standard. Despite the painstaking care of the researchers, the lack of scientific understanding and publicly available data about how nanosilica is absorbed in the gastro-intestinal system made it impossible to complete a risk assessment with the high degree of certainty required for standard-setting.
Limits to the effectiveness of Codex standards to protect consumer health
Any consideration of whether Codex standards and guidance texts on agri-nanotechnology will protect consumers is subject to at least three assumptions; 1.) that there is a sufficient quantity and quality of data and peer-reviewed studies from which to conduct a risk assessment about chronic gastro-intestinal exposure to ENMs; 2.) that the standards and guidance texts resulting from risk assessments are adopted by Codex member governments, implemented and enforced; 3.) that consumers are informed of the risks and benefits of incorporating ENMs in food, food packaging and food contact surfaces. Presently none of these assumptions are valid.
What motivations or pressures will begin to make these assumptions valid? Transnational agribusiness corporations deny that they are commercializing nano-foods.46 They have a strong motivation to protect their global brand reputations, and not to risk the economic value of those brands by trafficking nano-foods without some form of government legitimation. And yet, the FAO/WHO expert panel cited at the beginning of this essay, assumes the commercialization of some agri-nanotechnology products in some Codex member countries. The panel did not call for unregulated ENMs in food and food packaging to be pulled from the shelves.
An interim strategy for legal commercialization could be to seek agri-nanotechnology standards for generic food ingredients and food packaging materials not tied to a branded food. It is well possible that ENMs applied in generic foods, such as bananas, or to food packaging, could be in commerce for perhaps a decade before Codex agrees on a food additive standard for an ingredient in a food nanocoating. We assume that at some point in the near future, Codex will request FAO/WHO expert panels to risk assess a food additive, pesticide or veterinary medicine that incorporates ENMs. But until standards are publicly debated and agreed, consumers will be exposed to the possibility of chronically ingesting ENMs that will not receive government oversight unless there is an outbreak of acute toxicity that can be traced back to consumption of ENMs.
Whether the quality and quantity of peer-reviewed studies is sufficient to ensure a non-controversial and widely accepted standard cannot be forecast with any certainty. However, it does seem likely that a Codex standard or guidance text on ENMs in food packaging materials or on food contact surfaces could become the first Codex agri-nanotechnology product, particularly if peer-reviewed studies show that ENMs incorporated into food packaging do not migrate from the packaging material to the food packaged.
The implementation and enforcement fate of any agreed Codex standards or guidance texts is less certain. In 2005, the Commission, in deference to the WTO, decided to “abolish” (the formal term) its procedure for members to adopt Codex standards, since Codex members had stopped using the adoption procedure, and had begun to report their SPS measures to the WTO. Instead Codex members “proposed that the [Codex] Secretariat should work with the WTO Secretariat to consider how to monitor information on the use of Codex standards.”47 In practice, Codex standards and particularly guidance texts continue to influence the development of member countries’ standards, but currently there is no joint monitoring of the standards nor how they are used and enforced.
Instead, the SPS Agreement states that “Members shall accept the sanitary or phyto-sanitary measures of other Members as equivalent” (Article 4) either on a measure by measure basis or for entire fields of food safety, e.g., inspection and certification. Negotiations of bilateral “equivalence” agreements can be very controversial for either specific products or when an importing country makes SPS demands of the exporting country that are alleged to be trade discriminatory or not “based in science.” WTO members “notify” major new food safety laws to the WTO SPS Committee to inform other members and respond to member questions. This exchange of information may result in revisions to WTO member food safety laws, to avoid WTO trade dispute challenges to those standards.48
Given the subordination of Codex standards to trade rules and policy, can Codex standards help make agri-nanotechnology products safe for consumers? Codex member state positions are generally the result of a dialogue between food industry representatives and government officials, some of whom move into the food industry following government service. However, consumer organization interventions can be influential and even decisive, particularly if there is a disagreement among member governments. For example, the refusal of Codex to approve a ractopamine standard demanded by the United States on behalf of Dow Elanco, was due in part to the work of Consumers International.49
The Codex ideal was that standards developed using the best available science would be adopted by developing countries to improve their food safety and food security both domestically and in internationally traded food and agriculture products. There is a Codex fund to enable the participation of developing country delegates who otherwise might not be able to afford to go to the more than 30 Codex committee meetings a year. FAO and WHO have programs to advise, but not finance, developing country implementation of standards. However, FAO and WHO have been in budget crises for more than a decade, so the contribution of standards to protecting consumer health in developing countries is difficult to measure. The Codex Executive Committee proposal in February for FAO and WHO to adequately fund its joint expert committees for risk assessment50 reflects just one aspect of that funding crisis.
Codex standards are part of the rules that facilitate trade, along with customs rules, labeling rules, tariffs etc. If a WTO member blocks a food import, by using a standard which does not conform to the Codex standard, Codex standards may become commercial diplomacy tools used to overcome importing country resistance to a product without going through the expensive and lengthy WTO dispute settlement and authorized retaliation process. The barriers to using the SPS Agreement to block an imported food, plant or animal products are considerable.
“In cases where relevant scientific evidence is insufficient,” (Article 5.7) a WTO member may adopt a temporary SPS measure that could be used to justify an import refusal, but this measure must be reviewed “within a reasonable period of time” as more sufficient scientific evidence becomes available. However, a WTO member appeal to use precautionary measures, if there is scientific uncertainty or lack of data about a disputed product, will not find much support in Codex’s general principles. “Precaution is an inherent element of risk analysis” according to the ”Working Principles on Risk Analysis of Food Safety for Application by Governments,” 51 the result of a ten-year long battle among Codex members.
Because precaution is characterized as inherent, there is no specificity about the application of precautionary measures. The German government’s “Precautionary Strategies for Managing Nanomaterials,”52 for example, if applied to risk assessment of food with ENMs and if of longer duration than the temporary measures allowed by the SPS Agreement (Article 5.7), would not find a friendly welcome at Codex. Given the likely impending or existing commercialization of foods with ENMs, both Codex and the WTO will have to evolve to set relevant standards and use standards evidence that provides a wide berth for the uncertainties and insuffiency of scientific evidence about ENMs in food and food packaging materials.
Conclusion
Despite its limitations, Codex is the appropriate venue to begin to develop multilateral standards on this rapidly evolving technology. This article has surveyed the process of and a few challenges to Codex standard-setting for ENMs, both generic challenges and ones specific to nanosilica in food packaging. Even if there were more research and hence more publicly reviewable data on the gastro-intestinal effects of chronic exposure to inorganic ENMs, particularly metal oxides, it would not be easy to initiate, much less complete, standards and guidance texts on agri-nanotechnology. The difficulty does not reside simply in the subordination of Codex standards to the WTO SPS Agreement nor in the meager funding that FAO and WHO member governments provide for expert meetings53, and hence their infrequency.
Rather, nanotechnology poses systemic challenges to the numerical definitions of Codex standards, and their relevance to the protection of consumer health. For example, since the mass of an ENM is too small to provide a useful indicator of how much of a nano-metal oxide can bio-accumulate before it becomes a hazard to human health, then nano versions of all the metal oxide additives in the Codex list may need to be agreed. Revisions would also need to occur for Maximum Residue Levels for ENMs in veterinary drugs and pesticides. Guidance texts on food inspection and certification systems, on sampling and testing for agri-nanotechnology products, and on other issues likely would also require some revision.
The complex and comprehensive challenges that nanotechnology poses to risk assessment and standards setting argues for a decision by the Commission to include agri-nanotechnology standards work in its 2013-2018 Strategic Plan and, preferably, to form a Task Force on Agri-Nanotechnology. If Codex approaches agri-nanotechnology piecemeal, by responding to member requests for standards to legalize the commercialization of this or that product exported from a member country firm, then the likelihood of incoherence among standards and duplicative work among committees increases. A Task Force could decide public health, rather than commercial, priorities for agri-nanotechnology standard setting.
This process should be supported by the establishment of adequately funded FAO/WHO expert panels to assess the risks of food additives, pesticides or veterinary medicines that incorporate ENMs. The FDA and EPA could also contribute to this process by responding affirmatively to NGO petitions and lawsuits to mandate that agri-nanotechnology product developers submit data on their use of ENMs in currently commercialized products and products in a research and development stage. Analysis of such data will produce the peer-reviewed studies that the FAO/WHO joint expert bodies would review in the risk-assessment process.
Governments, as well as nanotech product developers, are using focus groups to determine which agri-nanotechnology products are most likely to be accepted by consumers.54 However, likelihood of consumer acceptance is an inadequate basis for developing a Codex work plan for nanotechnology, since focus-group findings are not a scientific basis for standards setting. Nor should governments develop agri-nanotechnology standards through a new organization, as has been recommended, to ensure consumer acceptance of agri-nanotechnology products.55
Evaluating the risk of harm to consumer health by consuming ENMs can only be accomplished through the risk assessments of product data that has yet to occur, for the most part, concerning the effects of ENMs on the gastro-intestinal system. Calls for moratoria on the commercialization of nanotechnology products prior to safety assessments have gone unheeded. Should Codex continue to wait for “other entities to complete their work” on regulating nanotechnologies and presumably set the terms for Codex to do so, when the “other entities” have just begun to move to get the data to initiate risk assessments? Or should Codex commit to include work on agri-nanotechnology standards and texts as part of its revised Strategic Plan for 2013-2018, regardless of what “other entities” do or don’t do?
Sometimes a political gesture is important, even from resource-deprived entities such as FAO, WHO and Codex. Since FAO and WHO acknowledge that some agri-nanotechnology products have entered commerce, it is imperative that to carry out Codex’s mandate of protecting consumer health, the next meeting of the Commission should commit to including agri-nanotechnology standards work in its Strategic Plan and to request the Codex Executive Committee to propose a work plan, schedule and budget for developing agri-nanotechnology standards and guidance tests. Committing Codex to work on agri-nanotechnology standards would be a politically and technically significant first step to put pressure on “other entities” to complete enough of their work to help enable Codex to face the many challenges of standard setting for agricultural and food applications of nanotechnologies.
Another FAO/WHO recommendation to wait until “other entities have completely their work” on regulating nano-technology could hold up the Codex process for years. Codex member governments, including those “other entities,” should move forward now with terms of reference and resources for a Task Force on agri-nanotechnology. Otherwise, we could be facing a very long wait, during which time agri-nanotechnology products commercialized without regulation could expose consumers to unnecessary and unjustifiable risks.
Endnotes
1 “FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications,” United Nations Food and Agriculture Organization, 2010, xvii. http://www.fao.org/ag/agn/agns/files/FAO_WHO_Nano_Expert_Meeting_Report_Final.pdf.
2 “Bananas,” InfoCom, UN Conference on Trade and Development, 2007. http://www.unctad.org/infocomm/anglais/banana/market.htm (accessed April 7, 2012).
3 “Nanoparticle size comparison,” Science Learning, June 29, 2008. http://www.sciencelearn.org.nz/Contexts/Nanoscience/Sci-Media/Images/Nanoparticle-size-comparison (accessed April 7, 2012).
4 Andrew Schneider, “Regulated or Not: Nano-foods Coming to a Store Near You,” AOL.News.com, March 24, 2010. http://www.aolnews.com/2010/03/24/regulated-or-not-nano-foods-coming-to-a-store-near-you/ (accessed April 3, 2012).
5 J.M. Lagarón et al., “Improving packaged food quality and safety: Part 2: Nanocomposites,” Food Additives and Contaminants, 22, no. 10 (October 2005): 994-998.
6 E.g. Rory Harrington, “Nano-coated ‘killer paper’ developed to extend food shelf life,” FoodProductionDaily.com, January 20, 2011. http://www.foodproductiondaily.com/content/view/print/353941 (accessed April 2, 2012).
7 Christina Kriegel et al, “Fabrication, Functionalization and Application of Electrospun Biopolymer Nanofibers,” Critical Reviews in Food Science and Nutrition 48, (2008):781.
8 Rory Harrington, “Is the food processing industry poised to embrace nanocoatings?” FoodProductionDaily.com, May 31, 2010. http://www.foodproductiondaily.com/Processing/Is-the-food-processing-industry-poised-to-embrace-nanocoatings (accessed March 23, 2012)
9 For a quick overview of food, feed and food packaging applications, see “What’s happening with nanofoods? Nanowerk, February 2, 2012. http://www.nanowerk.com/spotlight/spotid=24155.php (accessed March 23, 2012)
10 Georgia Miller and Rye Senjen, “Out of the Laboratory and On To Our Plates: Nanotechnology in Food and Agriculture,” Friends of the Earth Australia, Europe and U.S.A. (March 2008), Appendix 1. http://libcloud.s3.amazonaws.com/93/b5/4/547/Nanotechnology_in_food_and_agriculture_-_web_resolution.pdf
11 Schneider, “Regulated or Not: Nano-foods Coming to a Store Near You.”
12 Michael Taylor, “Assuring the Safety of Nanomaterials in Food Packaging: The Regulatory Process and Key Issues,” Project on Emerging Nanotechnologies, PEN 12 (June 25, 2008): 14. http://www.nanotechproject.org/publications/page2/ (accessed February 24, 2012).
13 See e.g. A Research Strategy for Environmental, Health and Safety Aspects of Engineered Nanomaterials, National Research Council of the National Academies of Science, (2012), 4. http://www.nap.edu/catalog.php?record_id=13347 (accessed February 23, 2012).
14 The White House, “Memorandum for the Heads of Executive Departments and Agencies,” Office of Science and Technology Policy, Office of Management and Budget and Office of the United States Trade Representative, June 9, 2011, 1. http://www.whitehouse.gov/sites/default/files/omb/inforeg/for-agencies/nanotechnology-regulation-and-oversight-principles.pdf (accessed January 25, 2012) and Environmental Protection Agency , “EPA Proposes Policy on Nanoscale Materials in Pesticide Products,” June 9, 2011. http://yosemite.epa.gov/opa/admpress.nsf/0/05FF063E9205EB3C852578AA005AA0F8 (accessed January 25, 2012).
15 International Center for Technology Assessment , “U.S. EPA Proposal Is First Step In Long Needed Regulation of Untested Nano-scale Pesticides Products,”, June 10, 2011.
16 Food and Drug Administration “Concerning Whether an FDA-Regulated Product Involves the Application of Nanotechnology,”June 9, 2011. http://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm (accessed January 25, 2012).
17 International Center for Technology Assessment, “Consumer Organizations File First Lawsuit on Risks of Nanotechnology,” December 21, 2011. http://www.icta.org/files/2011/12/Nano-JT-PR-vFinal-December-2011.pdf and International Center for Technology Assessment et al., “Complaint for Declaratory and Injunctive Relief,” December 21, 2011. http://www.centerforfoodsafety.org/wp-content/uploads/2011/12/1-Pls-Complaint.pdf (both accessed April 7, 2012).
18 “U.S., EU differ on Product Safety for Nanomaterials, Trade Fight Looms,” Inside U.S. Trade, October 9, 2009.
19 Codex Alimentarious Commission, “Standards,” 2011. http://www.codexalimentarius.org/standards/en/ (accessed February 28, 2012.)
20 Article 1, Statues of the Codex Alimentarius Commission, Procedural Manual, 20th edition, 2011 ftp://ftp.fao.org/codex/Publications/ProcManuals/Manual_20e.pdf.
21 For a register of SPS Agreement trade disputes, see World Trade Organization, “Disputes by agreement,” 2012. http://www.wto.org/english/tratop_e/dispu_e/dispu_agreements_index_e.htm?id=A19#selected_agreement (accessed April 1, 2011).
22 World Trade Organization, “Guidelines to further the practical implementation of Article 5.5,” Committee on Sanitary and Phytosanitary Measures, G/SPS/15, July 18, 2000.
23 Steve Suppan, “Codex standards and consumer rights,” Consumer Policy Review 16, no. 1 (January-February 2006): 5-13.
24 Codex Alimentarious Commission, “Statement of Principle Concerning the Role of Science in the Codex Decision-Making Process and the Extent to Which Other Factors Are Taken Into Account,” Decision of the 21st Session of the Commission, 1995. Procedural Manual, 20th edition, (2011) 209. ftp://ftp.fao.org/codex/Publications/ProcManuals/Manual_20e.pdf (February 20, 2012).
25 Consumers International, “Consumer rights victory as U.S. ends opposition to GM labeling guidelinesJuly 5, 2011. http://www.consumersinternational.org/news-and-media/news/2011/07/gm-labelling-victory-as-us-ends-opposition
26 Gregory Conko, “Shoppers already have a choice regarding biotech foods,” Food Chemical News, February 17, 2012.
27 NanoAction, “Principles for the Oversight of Nanotechnologies and Nanomaterials” (January 31, 2008), 8. http://www.icta.org/nanotechnology/publications-4/ (accessed March 27, 2012).
28 Stephen Clapp, “U.S. Codex manager sees “governance issues” looming this year,” Food Chemical News, January 13, 2012 and Clapp, “U.S. hopes to overturn opt-out provision in Codex additive standard,” Food Chemical News, February 17, 2012.
29 “FAO/WHO Expert Meeting . . .”, xviii.
30 Kenneth Chang, “Researchers Create Nanostructures, and Whip Up a Recipe, too,” The New York Times, September 7, 2010.
31 Rod Smith, “Food tech shows promise,” Feedstuffs, January 23, 2012. http://www.feedstuffsfoodlink.com/ME2/dirmod.asp?sid=&nm=&type=news&mod=News&mid=9A02E3B96F2A415ABC72CB5F516B4C10&tier=3&nid=C9D8B601F90F4CD28268EFBB523C47A0 (accessed March 12, 2012). The article summarizes Timothy V. Duncan, “The communication challenges presented by nanofoods,” Nature Nanotechnology, October 30, 2011. http://www.nature.com/nnano/journal/v6/n11/full/nnano.2011.193.html
32 See Steve Suppan “Racing Ahead: U.S. Agri-Nanotechnology in the Absence of Regulation,” Institute for Agriculture and Trade Policy, July 2011,http://www.iatp.org/files/2011.6.29%20AgriNanotech%20SS.pdf (accessed March 12, 2012).
33 European Commission, “Commission recommendation of a definition of a nanomaterial,” October 18, 2011. http://ec.europa.eu/environment/chemicals/nanotech/pdf/commission_recommendation.pdf (accessed February 23, 2012).
34 Peter Wick et al, “Barrier capacity of human placenta for nanomaterials,” Environmental Health Perspectives, 2009. http://www.ehponline.org/members/2009/0901200/0901200.pdf (accessed March 20, 2011)
35 European Environmental Bureau, “Nano definition too narrow, says EEB,” October 18, 2011. http://www.eeb.org/index.cfm/news-events/news/nano-definition-too-narrow-says-eeb/ (accessed February 23, 2012).
36 Corporate Europe Observatory, “EU officials going through Brussels’ revolving door to lobby industry,” December 6, 2011. http://www.corporateeurope.org/publications/eu-officials-going-through-brussels-revolving-door-lobby-industry-exposed (accessed February 24, 2011).
37 “EU food firms not rushing to use nano: Coca-Cola exec,” Food Chemical News, October 14, 2011.
38 Codex Alimentarious Commission, “Understanding the Codex Alimentarious” (2006), ftp://ftp.fao.org/codex/Publications/understanding/Understanding_EN.pdf (March 20, 2012).
39 Thomas G. Neltner et al, “Navigating the U.S. Food Additive Regulatory Program,” Comprehensive Reviews in Food Sciences and Food Safety 10, no.6: 342-368. http://onlinelibrary.wiley.com/doi/10.1111/j.1541-4337.2011.00166.x/full (accessed March 10, 2012).
40 Diagram of Codex committee structure, Procedural Manual, 20th edition (2011), 220. ftp://ftp.fao.org/codex/Publications/ProcManuals/Manual_20e.pdf (April 7, 2011)
41 Codex Alimentarius Commission, “Codex General Standard for Food Additives,” (2011), http://www.codexalimentarius.net/gsfaonline/docs/CXS_192e.pdf (April 7, 2011).
42 Stephen Clapp, “Codex inaction on ractopamine has immediate trade consequences,” Food Chemical News, July 15, 2011.
43 Susan Dekkers et al, “Presence and risks of nanosilica in food products,” Nanotoxicology, 2010, 1-13.
44 For a visual representation of in vitro digestion techniques, see Ruud Peters et al. Symposium on Recent Advances in Food Analysis, November 2011, “Nanoparticles in food and non-food: Recent methods and measurements,” http://www.nanolyse.eu/PublicDocs/OUTCOMES/L18_Peters.pdf (February 12, 2012)
45 Dekker et al, “Presence and risks of nanosilica in food products.” 4-5
46 E.g. “EU food firms not rushing to use nano: Coca-Cola exec,” Food Chemical News, October 14, 2011.
47 Codex Alimentarious Commission, “Report of the Twenty-Seventh Session of the Codex Alimentarius Commission,” ALINORM 05/28. July 4-9, 2005., paragraph 34. ftp://ftp.fao.org/codex/alinorm05/al28_41e.pdf (accessed March 25, 2012).
48 For a summary of issues raised and resolved in the SPS Committee, see “Specific Trade Concerns”, World Trade Organization, Committee on Sanitary and Phyto-sanitary Measures, G/SPS/GEN/204/Rev.11/Add.2, March 1, 2011. http://docsonline.wto.org/DDFDocuments/t/G/SPS/GEN204R11A2.doc (March 20, 2012).
49 Stephen Clapp, “Codex inaction on ractopamine has immediate trade consequences,” Food Chemical News, July 15, 2011.
50 “Codex Executive Committee seeks more funds for scientific panels,” Food Chemical News, February 17, 2012.
51 Codex Alimentarius Commission « Working Principles for Risk Analysis for Food Safety For Application by Govenrments, » (2007), Paragraph 12, CXG_062e.pdf, (accessed April 7, 2012).
52 German Advisory Council on the Environment, « Precuationary Strategies for Managing Nanomaterials : Summary for policymakers, » (September 2011). http://www.umweltrat.de/SharedDocs/Downloads/EN/02_Special_Reports/2011_09_Precautionary_Strategies_for_managing_Nanomaterials_KFE.pdf?__blob=publicationFile (accessed April 7, 2011).
53 Codex Alimentarious Commission, “Report of the Sixty-Fourth Session of the Executive Committee of the Codex Alimentarius Commission,” June 29–July 2, 2010, ftp://ftp.fao.org/codex/Alinorm10/al33_03Ae.pdf. (accessed March 23, 2011).
54 E.g. Food Standards Agency (United Kingdom), “FSA Citizens Forums: Nanotechnology and food,” April 2011, 4. http://www.food.gov.uk/multimedia/pdfs/publication/fsacfnanotechnologyfood.pdf (accessed March 27, 2012).
55 Sarah Hills, “GM: a “cautionary tale” for nanotechnology,” FoodNavigator.com, March 7, 2012. http://www.foodnavigator.com/Financial-Industry/GM-a-cautionary-tale-for-nanotechnology (accessed March 27, 2012).