FDA approves eyesight-enhancing technology
March 21, 2011
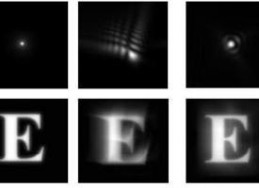
A dot and an "E" showing contrast between perfect vision (left) and aberrations (Photo: University of Rochester Medical Center)
The FDA has approved the use of a refractive surgery technology called the Rochester Nomogram, says Scott MacRae, M.D., who helped develop the formula at the University of Rochester Medical Center.
With the aid of the Nomogram, 99.3 percent of the eyes that MacRae operates on using LASIK surgery have vision of 20/20 or better. The Nomogram was first created and tested about five years ago by MacRae working together with Manoj Venkiteshwar, Ph.D.
Venkiteshwar and MacRae helped to create a field known as customized ablation, a form of LASIK that corrects subtle imperfections, bringing about a super-crisp quality of eyesight. Beyond making vision on the order of 20/15 or 20/16 possible or even commonplace in some groups of patients, the technology also increases the eye’s ability to see in situations where there is low light or little contrast.
They calculated the subtle effects that customized ablation can have on the eye and how the eye shunts around light. Specifically, they found that fixing subtle imperfections that hadn’t even been recognized before offered new opportunities for enhancing a person’s vision. They developed the Rochester Nomogram to allow surgeons to take advantage of this information during refractive surgery.
The FDA approval is the latest development in a nearly 20-year-long project by University of Rochester scientists and physicians to study and improve human vision, explains MacRae. MacRae is the author of two best-selling books on customized ablation, including Customized Corneal Ablation: The Quest for Supervision.
Full disclosure: Dr. MacRae was my ablation surgeon. – AA.