Combating Antibiotic Resistance: Ineffective Drugs Push Scientists To Explore Alternative Medications
They were once the darlings of modern medicine. With them, surgery could be clean and safe, and infections of all kinds treated quickly and relatively cheaply. But as two deaths at a University of California, Los Angeles hospital linked this week to a highly resistant infection show, antibiotics are no longer as effective as they used to be. While the California cases and similar ones elsewhere renew public interest in antibiotic resistance, scientists are trying to create ways to prepare for the day when antibiotics no longer work.
Scientists say there are promising options on the horizon to treat infections that are immune to standard antibiotics. A handful of new antibiotics are under development, as are other methods of combating infections that target bacteria differently from the way antibiotics do. But that horizon is distant, and many harmful bacteria already have outsmarted current methods to kill them.
“We are way behind the curve on this,” Dr. Peg Riley, who runs and researches microbial evolution at the Riley Lab at the University of Massachusetts Amherst, said of medical developments to deal with antibiotic resistance. “That’s because [the government] stopped funding antibiotic development, and pharmaceutical companies stopped funding it because they thought they had the battle won.”
Certain infections, like ones caused by carbapenem-resistant Enterobacteriaceae, or CRE, which was found at UCLA, are more of a threat to sick individuals in hospitals and other facilities than to the general population. But other more easily communicable infections are becoming increasingly dangerous as the bacteria that cause them develop immunity. The U.S. Centers for Disease Control and Prevention (CDC) estimates that every year more than 2 million people in the U.S. are infected with resistant bacteria and that at least 23,000 die as a result.
Riley is one of the world’s foremost experts on bacteriocins, toxic proteins produced by bacteria to fight off other similar bacteria. She works with Pheromonicin Biotech Ltd., a company in Beijing, to experiment with and cultivate ways to use these proteins to target and kill specific pathogens, unlike traditional antibiotics, which kill bacteria, good and bad, rather indiscriminately. Good bacteria are essential for staying healthy, and Riley called bacteriocin-based drugs “a more intelligent approach to dealing with those relatively few pathogens that actually cause us trouble.”
The Chinese government is investing $400 million annually in this research, according to Riley, but pharmaceutical companies aren’t that interested in them. “They’re very powerful,” she said of bacteriocins, “but they’ve not been in the mainstream in drug development.” She couldn’t say when some of the drugs that she and her Chinese counterparts are engineering might become available. None have even entered human trials yet, but she said that of all the research into medicines that could treat resistant pathogens, drugs using bacteriocins might be available the soonest.
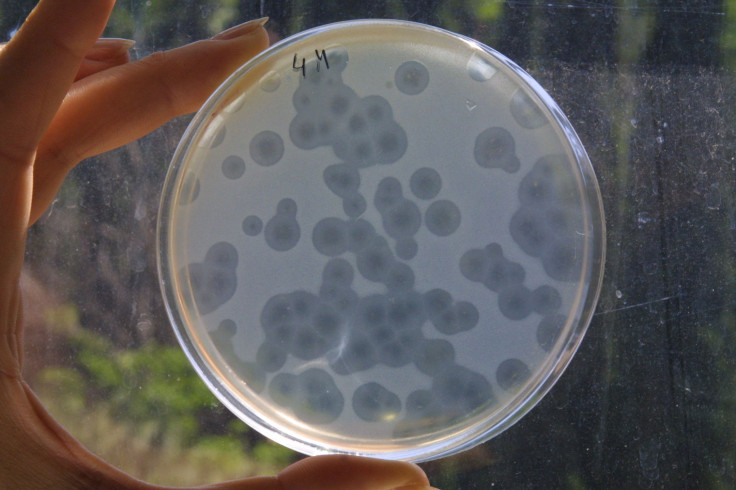
Another possibility to treat resistant pathogens is the use of bacteriophages, which are viruses that infect and kill bacteria. Like bacteriocines, they target specific bacteria instead of killing all bacteria they come into contact with, the way antibiotics do. Countries in the former Soviet Union have long used bacteriophage therapy to kill pathogens, while Western countries were focused on using antibiotics. But now that antibiotic resistance is growing, other countries have started to take a greater interest in the possibilities of bacteriophage therapy.
But using bacteriophages to kill pathogens is far from full-fledged therapy. “It’s quite basic research at the moment,” said Dr. Rob Meijers, who studies bacteriophages at the European Molecular Biology Laboratory in Hamburg, Germany. Although such therapies were used in the Soviet Union, “it was sort of a haphazard approach, because sometimes it worked and sometimes it didn’t.”
What Meijers and his fellow researchers are trying to figure out is how bacteriophages that kill one particular strain of bacteria, Clostridium difficile, actually work. Bacteriophages will kill bacteria, Meijers explained, but it won’t eradicate them entirely, because otherwise the phages themselves cannot survive. “It won’t immediately start killing everything. We are trying to find out how this is regulated,” he said. Still, he emphasized, “This [research] is, at the moment, at the stage where we are just trying to understand how this works.”
Although bacteriophages seem promising in the distant future, after their mechanisms are better understood and can be controlled, commercializing drugs that make use of them also has many obstacles. Because phage therapy has been used for the past century, patenting it is difficult. It’s also expensive, especially when compared with antibiotics, Meijers said. “This is not something that will suddenly now cure the problem of antibiotic-resistant bacteria,” he said. “I don’t think it will replace antibiotics, but using both in combination could be very powerful.”
The so-called drug pipeline also has some new antibiotics aimed at fighting resistant bacteria. According to a brief from the Pew Charitable Trusts, as of last September, 38 new antibiotics aimed at superbugs were in clinical trials or had submitted applications to the Food and Drug Administration for approval. The number might seem high, but the brief was quick to point out that only 20 percent of drugs tested in humans are ultimately approved by FDA.
One reason the number of new antibiotics is so limited is that pharmaceutical companies have little financial incentive to invest in them. Drugs that treat chronic diseases are far more profitable than antibiotics, which are taken for a limited time. As UMass’ Riley noted, “How can you argue to your investors to invest $1 billion in a drug that you hopefully won’t have to use very often?” Most of the companies trying to develop the drugs noted in the Pew brief were small, with just four of the 29 companies coming from the top 50 pharmaceutical companies in sales.
Last month, scientists discovered an antibiotic found to be effective against several highly resistant pathogens. They called it teixobactin, and its discovery unleashed a spate of media coverage and praise as a sort of miracle antibiotic. But Dr. Kim Lewis, lead researcher of the team that discovered the antibiotic, has estimated it would be at least another two years before the drug hits human trials and another five before it could be used in a medical setting.
© Copyright IBTimes 2024. All rights reserved.












